Research
The brain is made up of precisely wired neuronal circuitry with assemblies of neuronal cells that continually receive information, elaborate and perceive it, and make decisions. The neuronal cells use chemical substances called neurotransmitters to mediate most of the synaptic transmission in the nervous system. Synaptic transmission occurs at synapses, where the axon comes in contact with other neurons and passes information on to them. Hence proper axonal growth to appropriate synaptic targets is essential for the production of a functioning nervous system. We are interested in understanding the molecular and cellular mechanisms of the interaction between the neurotransmitters and their receptors, and the signal transduction underlying axonal guidance, transport, and synaptic plasticity.
Project: The role of DOPA as a neurotransmitter
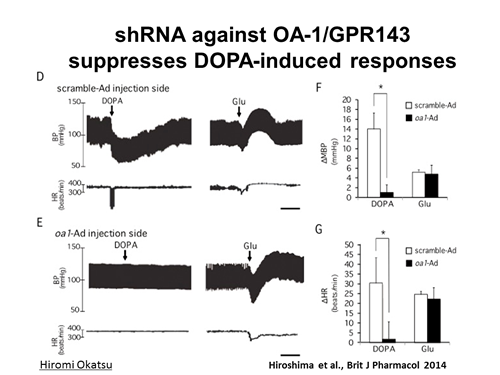
Searching for the neurotransmitters and their receptors is on of the most important issues in the filed of neuroscience. L-3,4-dihydroxyphenylalanine (DOPA) is a precursor of a neurotransmitter dopamine in the central nervous system. DOPA is a gold standard replenishing therapy for patients affected with Parkinson’s disease (Figure 1). DOPA is readily converted to dopamine by aromatic L-amino acid decarboxylase. Since 1986 we have proposed that DOPA is a neurotransmitter in the central nervous system. It is released from neurons, which may contain DOPA as an end product. DOPA itself produces pre- and postsynaptic responses. Furthermore, DOPA is also a possible candidate as a neurotransmitter for baroreflex in nucleus tractus solitarii (NTS) in the brain stem. Competitive DOPA antagonists antagonize all specific receptor(s) for DOPA, which mediates pharmacological responses to DOPA itself. If specific receptors for DOPA exist, could it be a promising drug target for Parkinson’s disease and other diseases? We’re now focusing on the projects to identify specific DOPA receptors. We recently identified GPR143 as a receptor for DOPA, which mediates depressor and bradycardic response to DOPA in the NTS (Figure 2). By establishing Gpr143-deficient mice, we are now performing phenotypic analysis, hoping to understand physiological and pathophysiological roles of DOPAergic system.
Funding sources: Grants-in-Aid for Scientific Research (B) from the Ministry of Education, Culture, Sports, Science and Technology, Japan.

Project: Signal transduction mechanisms of Semaphorins
Through screening of chemical reagents and studies of biochemistry, genetics and live cell imaging, we are trying to understand the molecular mechanisms underlying the regulation of axon/dendrite patterning, cell migration and synapse formation by axon guidance molecules such as Semaphorins and Netrins. In this study, we are particularly interested in the study of a family of proteins called Collapsin Response Mediator Proteins (CRMPs). CRMP family consists of five intracellular phosphoproteins (CRMP1 to CRMP5 and their splicing variants) of similar molecular size and high amino acid sequence identity. Each CRMP shows characteristic tissue distribution, and may have distinct functions, most of which are largely unknown. We have identified CRMPs as intracellular proteins that mediate Semaphorins downstream signaling. However, the mechanisms of CRMPs remain to be elucidated.
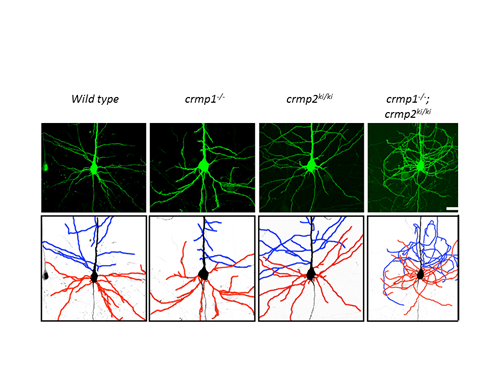
We discovered that CRMP2 is a physiological substrate for cyclin-dependent kinase 5 (Cdk5), which acts as a downstream mediator of Semaphorin3A (Sema3A)-induced growth cone collapse. In crmp1-/-; crmp2S522A knock-in mutant (crmp2ki/ki) mice, dendrites of layer V pyramidal neurons shows “curling phenotype”, thereby showing that CRMP1 and CRMP2 synergistically regulate dendritic patterning (Figure 1).
Project: The role of CRMP family proteins in the pathogenesis of Alzheimer’s disease
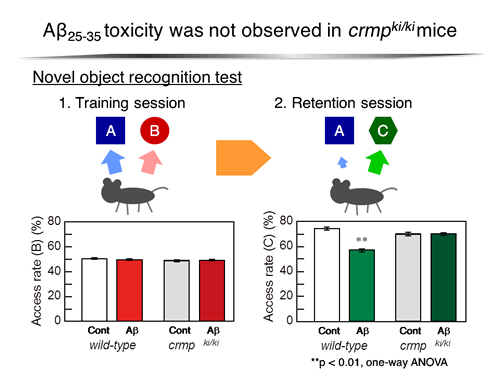
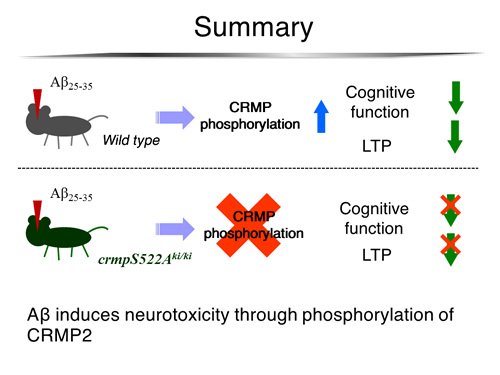
Topics in pathophysiology of axon guidance and synapse maturation have now attracted many investigators as an important research field in neuroscience. 3F4, the monoclonal antibody for the phosphorylated form of CRMP2, strongly labeled neurofibrillary tangles and plaque neurites (Yoshida et al., J Biol Chem, 1998). Our question is whether the accumulation of phosphorylated form of CRMP2 is causally related to progressive neuronal degeneration and/or neuronal cell death in Alzheimer's disease. In fact, CRMP family proteins are closely related to various kinds of pathophysiological disorders such as Alzheimer’s disease (Figures 2 and 3).
Project: The physiological roles of axonal transport elicited by axon guidance molecules
Besides functioning as repellents for navigating axons during neuronal circuit development, recent studies have implicated Semaphorins, the extracellular guidance molecules, in other events of neuronal development such as axonal fasciculation, dendritic maturation and axonal transport. In this study, we investigate the physiological relevance of axonal transport elicited by Sema3A. Using computer-assisted and video-enhanced differential interference contrast microscopy, we have demonstrated that Sema3A potently promotes axonal transport through neuropilin-1, a component of Sema3A receptor complex, in axonal growth cone. Our recent observation indicated that Sema3A-induced axonal transport is positively correlated to local protein synthesis monitored by immunofluorescence levels of phsphorylated eukarotic translation initiation factor 4E (eIF-4E) in growth cones. Since CRMPs are related to UNC-33, a nematode neuronal protein required for proper axonal extension and mutations of UNC-33 affect neural microtubules, the basic cytoskeletal elements for axonal transport. We also investiate the roles of CRMPs in Sema3A-induced axonal transport. We are now obtaining evidence that CRMPs may be involved in Sema3A-induced axonal transport that plays a crucial role in dendritic patterning and synapse maturation.
Funding sources: Grants-in-Aid for Scientific Research in a Priority Area from the Ministry of Education, Science, Sports and Culture, Japan and Core Research for Evolutional Science and Technology of the Japan Science and Technology Corporation
Project: The role of PTPase (protein tyrosine phosphatases) in developing and adult nervous system
PTPase are expressed in the central nervous system (CNS) as well as peripheral neurons and organs. However, their physiological functions are largely unknown. We recently obtained evidence that, PTP, one of the receptor type IIa protein tyrosine phosphatase localizes in axons as well as in dendrite to regulate their elaboration in the CNS.
Funding sources: Grants-in-Aid for Scientific Research (C) from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
Project: Studies on localization of axon guidance molecules and their receptors in C. elegans
Using C. eleganse as the model organisms, we are interested in the underlying mechanisms, which contribute to the localization of several axon guidance melecules (UNC6/Netrin, SLT-1/Slit ...etc) and their receptors (UNC5, SAX-3/Robo ...etc). Specifically, UNC-51 and UNC-14 are required for the localization of UNC-6/Netrin and UNC-5. In addition, we are analyzing mutations that show defects in the localization of axon guidance molecules and their receptors. This study will provide new insights toe the field of axon guidance.
Funding sources: Grants-in-Aid for Scientific Research (C) from the Ministry of Education, Culture, Sports, Science and Technology, Japan.


