Research
Astringency loss in kaki (Diospyros kaki)
Astringency is a unique character of kaki fruit. We are studying the physiology and genetics of astringency loss in kaki. Our results are used to breed PCNA (pollination-constant and non-astringent)-type kaki, the fruit of which is most desirable for fresh consumption. We have developed several molecular markers for selecting PCNA seedlings. Furthermore, we are now surveying kaki germplasm in China to find novel genetic resources for PCNA-type kaki.

Figure 1 Chinese non-astringent cultivar, ‘Ruo-Tian-Tian-Shi’
References
- Kanzaki, S., T. Akagi, T. Masuko, M. Kimura, M. Yamada, A. Sato, N. Mitani, N. Utsunomiya, and K. Yonemori. 2010. SCAR markers for practical application of marker-assisted selection in persimmon (Diospyros kaki Thunb.) breeding. J. Japan. Soc. Hort. Sci. 79: 150-155.
- Akagi, T., R. Tao, T. Tsujimoto, A. Kono, and K. Yonemori. 2012. Fine genotyping of a highly polymorphic ASTRINGENCY-linked locus reveals variable hexasomic inheritance in persimmon (Diospyros kaki Thunb.) cultivars. Tree Genetics & Genomes 8:195-204.
Self-incompatibility in Prunus (Rosaceae)
Most Prunus (Rosaceae) fruit tree species, including almond, sweet cherry, Japanese apricot, and plum, exhibit a homomorphic gametophytic self-incompatibility system in which the specificity of self/nonself-recognition is controlled by products encoded within the S locus. During pollination, a self-incompatibility (SI) reaction is triggered when the same ‘S allele’ specificity is expressed in both the pollen and pistil. We have succeeded in identifying the pistil S determinant (S-RNase) and pollen S determinant (F-box protein, SFB) genes and in developing PCR-based S genotyping and a marker-assisted selection system for self-compatible individuals. We are currently working to elucidate the distinct molecular recognition mechanism of the SI system in Prunus.

Figure 1 2D-PAGE of stylar proteins of sweet cherry
References
- Yamane, H. and R. Tao. 2009. Molecular basis of self-(in)compatibility and current status of S-genotyping in rosaceous fruit trees. J. Japan. Soc. Hort. Sci. 78: 137-157.
- Tao, R. and A.F. Iezzoni. 2010. The S-RNase-based gametophytic self-incompatibility system in Prunus exhibits distinct genetic and molecular features. Scientia Horticulturae 124: 423-433.
Bud dormancy in temperate fruit trees
Bud dormancy in deciduous fruit tree species is a complex process necessary for plant survival in unfavorable environments. Once formed in the summer, buds enter an endodormant state and require a certain period of cold temperatures to resume growth. Recent global warming may thus cause serious problems such as irregular or absent flowering. We are trying to find the internal factors or external signals controlling endodormancy. Currently, we are focusing on transcription factors that exhibit chilling requirement–dependent expression patterns.
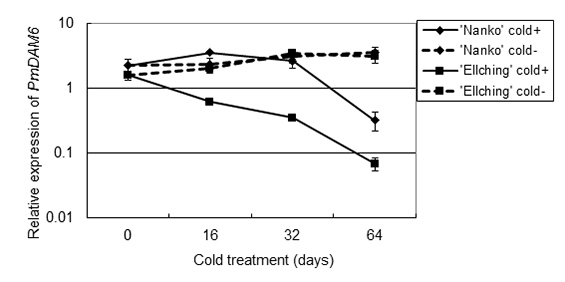
Figure 1 Transcription factor, PmDAM6 was down-regulated in genotype-specific chilling requirement-dependent manner. ‘Nanko’: Middle blooming genotype, ‘Ellching’: Early blooming genotype.
References
- Yamane, H. (2014) Regulation of bud dormancy and bud break in Japanese apricot (Prunus mume Siebold & Zucc.) and peach [Prunus persica (L.) Batsch]: A summary of recent studies. J. Japan. Soc. Hort. Sci. 83: 187-202.
Sexuality regulation
Sexuality, a fundamental mechanism for maintaining genetic diversity in a species, is one of the most important traits in both the breeding and cultivation of a crop. The sex-determination systems of plants, however, have been exploited in relatively few species, and particularly little is known regarding dioecious (showing distinct male and female individuals) sex-determination mechanisms. We are attempting to uncover the sex-determination system in oriental persimmon (Diospyros kaki) and its wild relatives (Diospyros spp.), cultivars of which have been maintained in our orchard since the establishment of our lab in 1926.
The figure shows a working model for the sex-determination system with OGI (Oppressor of meGI) and MeGI (Male Growth Inhibitor) genes in Diospyros species. OGI, which is located on the Y chromosome, makes small RNAs and triggers transitive RNAi against MeGI transcripts, while MeGI represses androecium development.
Sex expression in plants is flexible and has changed with polyploidization and/or domestication. Maleness expression is determined by OGI genotype in cultivated oriental persimmon (D. kaki), as occurs in other Diospyros species. The sex expression of this species, however, is more flexible and complicated. We are currently studying to identify underlying mechanisms of flexible sex determination. At the same time, we are studying sex determination in other tree crops, such as kiwifruit and Vitis spp., to clarify the diversity found among sex-determination systems in tree crops.
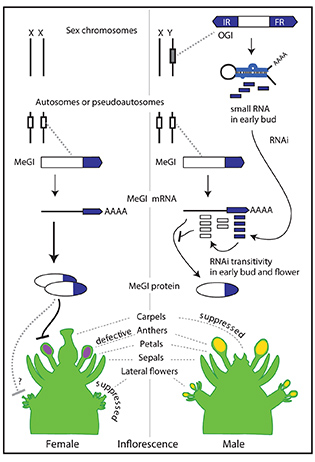
Figure 1 Chromosomal map of OGI and MeGI with model of their interaction and potential function in female (left) and male (right) flower development. OGI, which is located on the Y-chromosome, can trigger transitive RNAi in MeGI. On the other hand, MeGI acts as feminizing gene.
References
- Akagi, T., I. M. Henry, R. Tao and L. Comai. 2014. A Y-chromosome-encoded small RNA acts as a sex determinant in persimmons. Science 346: 646-650.
- Akagi, T., K. Kajita, T. Kibe, H. Morimura, T. Tsujimoto, S. Nishiyama, T. Kawai, H. Yamane, and R. Tao. 2014. Development of molecular markers associated with sexuality in Diospyros lotus L. and their application in D. kaki Thunb. J.Japan. Soc. Hort. Sci. 83: 214-221.
Genetic transformation in fruit tree species
Fruit tree breeding is often hindered by long generation times, large plant sizes, and reproduction barriers such as self-sterility, self-incompatibility, dioecy, etc. The same issues also complicate the evaluation of the function of unknown genes isolated from molecular genetic studies. We are developing novel breeding and gene evaluation techniques based on transgenic technologies such as agrobacterium-mediated gene transfer and virus-induced gene silencing (VIGS).

(Left) PDS-silenced VIGS seedling of sweet cherry, (right) control
References
- Kawai, T., A. Gonoi, M. Nitta, M. Kaido, N. Yamagishi, N. Yoshikawa, and R. Tao. 2014. Virus-induced gene silencing in apricot (Prunus armeniaca L.) and Japanese apricot (P. mume Siebold & Zucc.) with the Apple Latent Spherical Virus vector system. J. Japan. Soc. Hort. Sci. 83:23-31.
- Tetsumura, T., E. Giordani, and R. Tao. 2008. Persimmon (Kaki), p. 235-258. In: C. Kole and T. C. Hall (eds.). Compendium of Transgenic Crop Plants: Transgenic Tropical and Subtropical Fruits and Nuts. Blackwell Publishing, Oxford.
Regulation of flowering in fruit trees
The juvenile period typically lasts several years in fruit trees. Pomelo, however, has an ability called precocious flowering, which allows the plant to bear terminal flowers a year after seed germination with exposure to cool temperatures in the autumn. Furthermore, some cultivars of several fruit tree species have the perpetual flowering habit, although most temperate fruit trees bloom only once in an annual growing season, an adaptation of the growth cycle to seasonal environmental changes. We are attempting to understand the regulatory mechanisms of flowering in fruit trees by focusing on these phenomena.
References
- Esumi, T., Y. Kitamura, C. Hagihara, H. Yamane, and R. Tao. 2010. Identification of a TFL1 ortholog in Japanese apricot (Prunus mume Sieb et Zucc.). Scientia Hort. 125: 608-616.

Southern-highbush blueberry which blooms in spring and autumn
Evolutionary genomics
The characteristics of tree crops, such as their long juvenile phases (generation time) and highly heterogeneous genomes, make the application of common genetic techniques totally dependent on crossing and mutagenesis. We are now examining various existing cultivars and species in the Prunus or Diospyros genera to assess the genomic structures and important loci under selection in their evolutionary paths. We are exploiting bioinformatics approaches and whole-genome-wide techniques for fruit trees.
References
- Arus, P., T. M. Gradziel, M. M. Oliveira, and R. Tao. 2009. Genomics of almond, p.187-219. In: K.M. Folata and S.E. Gardiner (eds.). Genetics and Genomics of Rosaceae, Plant Genetics and Genomics: Crops and Models 6. Springer, Berlin, Heiderberg.
Other
We are investigating the commercial usages of a new persimmon variety, “Baby-persimmon,” that bears small fruits. We are also studying plant hormonal controls of important agronomic traits.

(Right) ‘Hiratanenashi’,
(Left) ‘Totsutanenashi (also known as baby-persimmon)
(bud sport mutant of ‘Hiratanenashi’)
References
-
Yamane, H., M. Ichiki, R. Tao, T. Esumi, K. Yonemori, T. Niikawa, and H. Motosugi. 2008. Growth characteristics of a small-fruit dwarf mutant arising from bud sport mutation in Japanese persimmon (Diospyros kaki Thunb.). HortScience 43: 1726-1730.